A New Lamellar Gold Thiolate Coordination Polymer, [Au(m-SPhCO2H)]n, for the Formation of Luminescent Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [Au(m-SPhCO2H)]n
2.3. Synthesis of the Polymer Composites
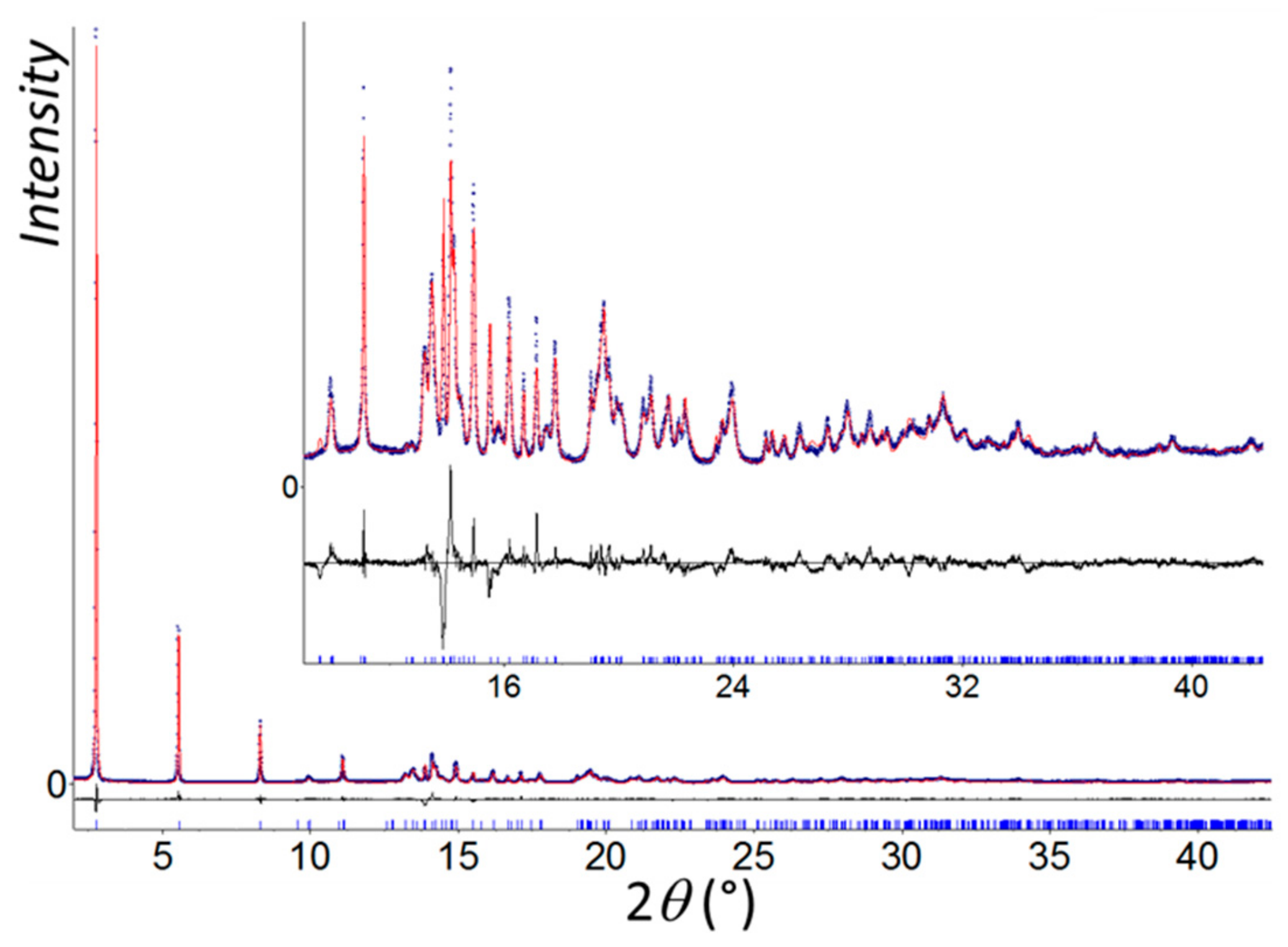
2.4. Structure Determination
2.5. Characterizations
3. Results and Discussion
3.1. Synthesis and Characterization
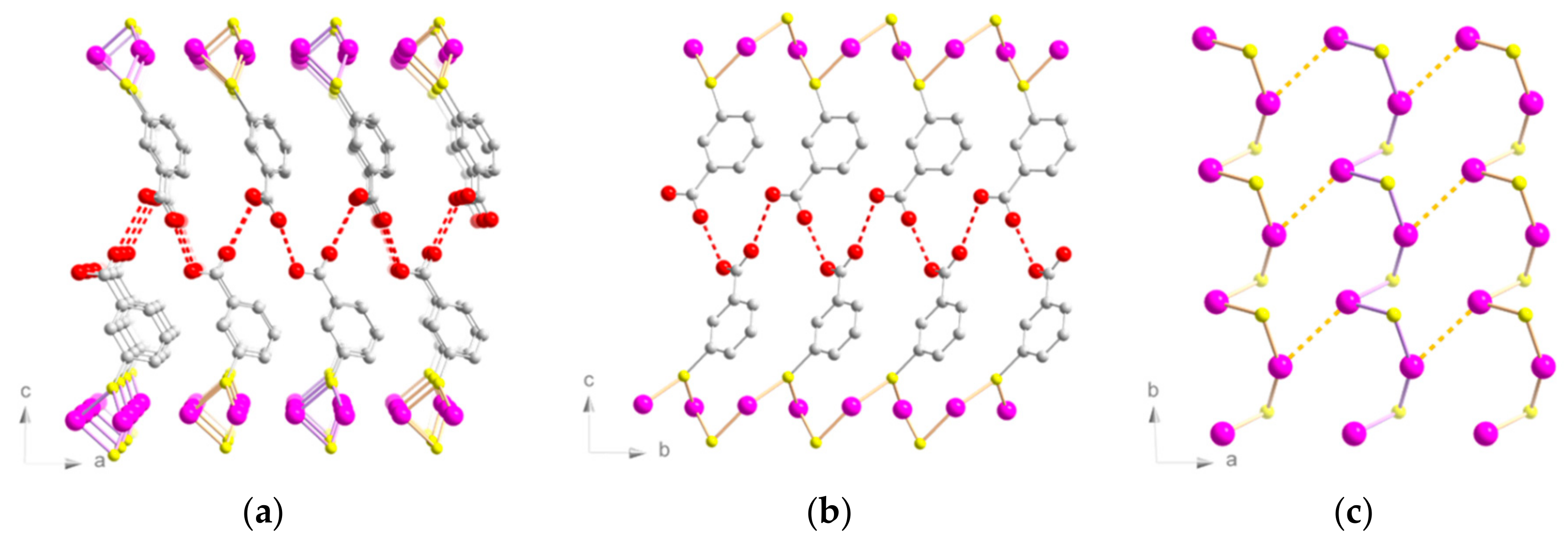
3.2. Structure
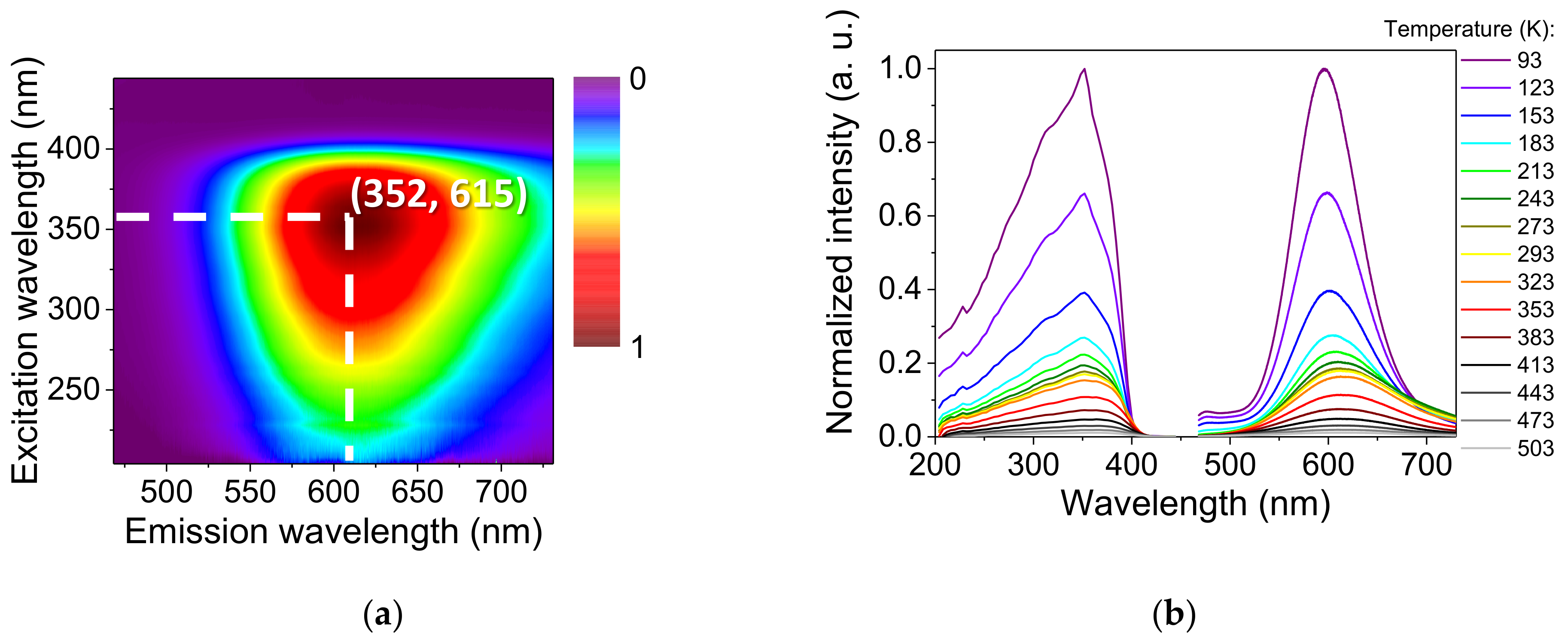
3.3. Photophysical Properties
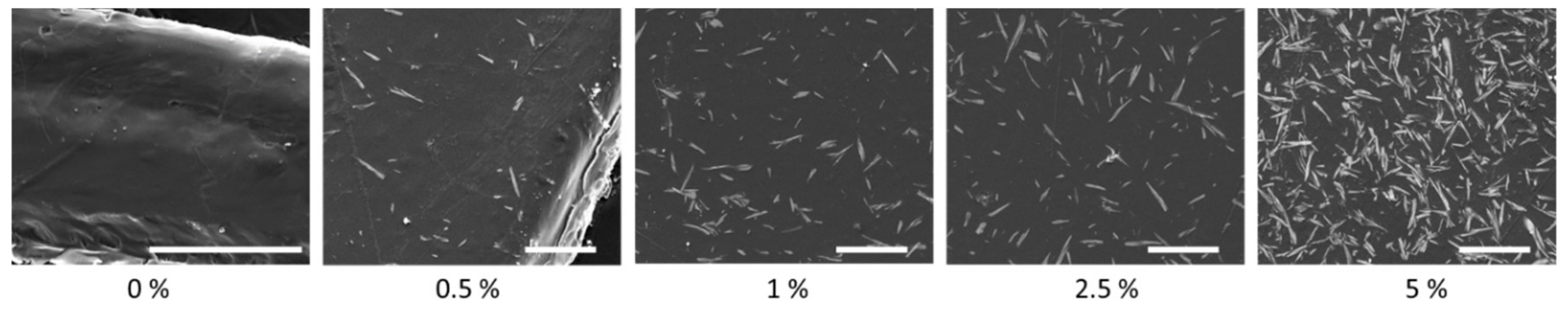
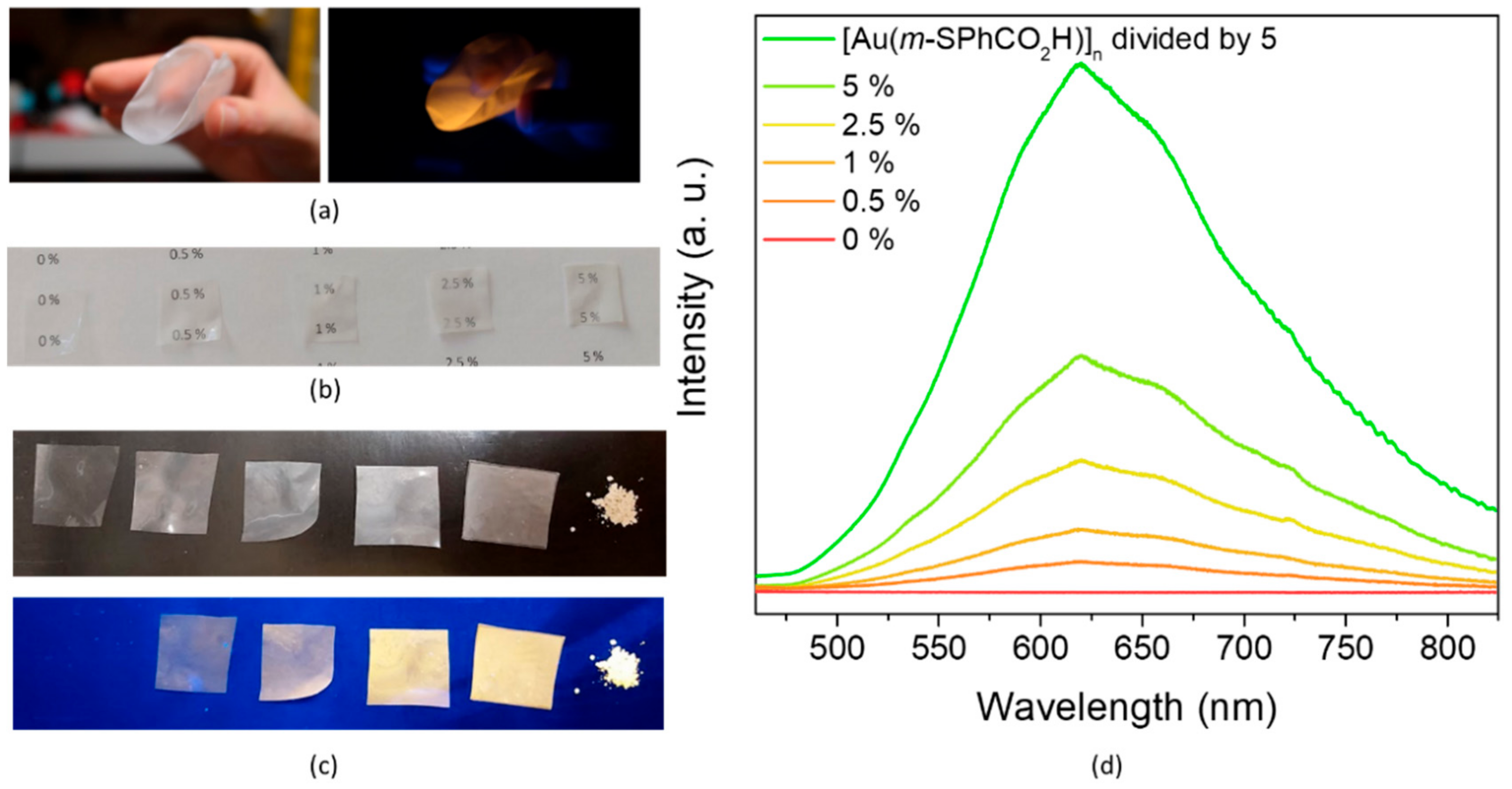
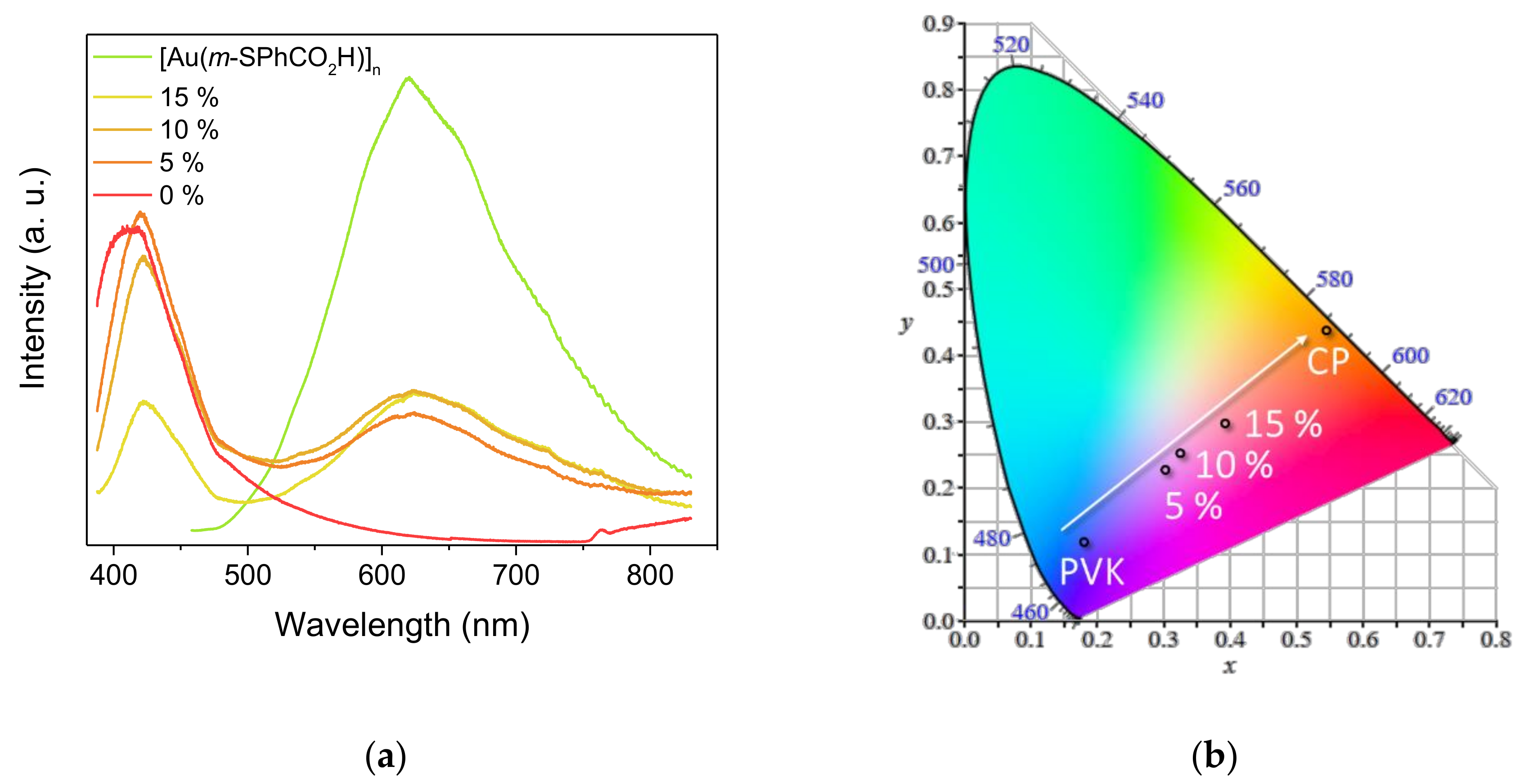
3.4. Polymer Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yuan, X.; Chen, T.; Leong, D.T.; Xie, J. Engineering Functional Metal Materials at the Atomic Level. Adv. Mater. 2018, 30, 1802751. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, M. Intra-cluster growth meets inter-cluster assembly: The molecular and supramolecular chemistry of atomically precise nanoclusters. Coord. Chem. Rev. 2019, 394, 1–38. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Song, X.-R.; Goswami, N.; Yang, H.-H.; Xie, J. Functionalization of metal nanoclusters for biomedical applications. Analyst 2016, 141, 3126–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au10−12(SG)10−12 Nanomolecules for High Tumor Specificity and Cancer Radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Chen, T.; Du, Y.; Ge, H.; Zhang, B.; Xie, J.; Yu, H.; Zhu, M. Design and mechanistic study of a novel gold nanocluster-based drug delivery system. Nanoscale 2018, 10, 10166–10172. [Google Scholar] [CrossRef]
- Cantelli, A.; Guidetti, G.; Manzi, J.; Caponetti, V.; Montalti, M. Towards Ultra-Bright Gold Nanoclusters. Eur. J Inorg. Chem. 2017, 2017, 5068–5084. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, M.; So, W.Y.; Huang, J.; Li, M.; Kauffman, D.R.; Cotlet, M.; Higaki, T.; Peteanu, L.A.; Shao, Z.; et al. A Mono-cuboctahedral Series of Gold Nanoclusters: Photoluminescence Origin, Large Enhancement, Wide Tunability, and Structure–Property Correlation. J. Am. Chem. Soc. 2019, 141, 5314–5325. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Lavenn, C.; Okhrimenko, L.; Guillou, N.; Monge, M.; Ledoux, G.; Dujardin, C.; Chiriac, R.; Fateeva, A.; Demessence, A. A luminescent double helical gold(i)–thiophenolate coordination polymer obtained by hydrothermal synthesis or by thermal solid-state amorphous-to-crystalline isomerization. J. Mater. Chem. C 2015, 3, 4115–4125. [Google Scholar] [CrossRef]
- Lavenn, C.; Guillou, N.; Monge, M.; Podbevšek, D.; Ledoux, G.; Fateeva, A.; Demessence, A. Shedding light on an ultra-bright photoluminescent lamellar gold thiolate coordination polymer [Au(p-SPhCO2Me)]n. Chem. Commun. 2016, 52, 9063–9066. [Google Scholar] [CrossRef] [PubMed]
- Veselska, O.; Okhrimenko, L.; Guillou, N.; Podbevšek, D.; Ledoux, G.; Dujardin, C.; Monge, M.; Chevrier, D.M.; Yang, R.; Zhang, P.; et al. An intrinsic dual-emitting gold thiolate coordination polymer, [Au(+I)(p-SPhCO2H)]n, for ratiometric temperature sensing. J. Mater. Chem. C 2017, 5, 9843–9848. [Google Scholar] [CrossRef]
- Veselska, O.; Podbevšek, D.; Ledoux, G.; Fateeva, A.; Demessence, A. Intrinsic triple-emitting 2D copper thiolate coordination polymer as a ratiometric thermometer working over 400 K range. Chem. Commun. 2017, 53, 12225–12228. [Google Scholar] [CrossRef] [PubMed]
- Veselska, O.; Dessal, C.; Melizi, S.; Guillou, N.; Podbevšek, D.; Ledoux, G.; Elkaim, E.; Fateeva, A.; Demessence, A. New Lamellar Silver Thiolate Coordination Polymers with Tunable Photoluminescence Energies by Metal Substitution. Inorg. Chem. 2019, 58, 99–105. [Google Scholar] [CrossRef]
- Gussenhoven, E.M.; Fettinger, J.C.; Pham, D.M.; Balch, A.L. A Reversible Polymorphic Phase Change Which Affects the Luminescence and Aurophilic Interactions in the Gold(I) Cluster Complex, [μ3-S(AuCNC7H13)3](SbF6) [J. Am. Chem. Soc. 2005, 127, 10838–10839. [Google Scholar] [CrossRef]
- Cha, S.-H.; Kim, J.-U.; Kim, K.-H.; Lee, J.-C. Preparation and Photoluminescent Properties of Gold(I)−Alkanethiolate Complexes Having Highly Ordered Supramolecular Structures. Chem. Mater. 2007, 19, 6297–6303. [Google Scholar] [CrossRef]
- Veselska, O.; Demessence, A. d10 Coinage Metal Organic Chalcogenolates: From Oligomers to Coordination Polymers. Coord. Chem. Rev. 2018, 355, 240–270. [Google Scholar] [CrossRef]
- Troyano, J.; Castillo, O.; Martínez, J.I.; Fernández-Moreira, V.; Ballesteros, Y.; Maspoch, D.; Zamora, F.; Delgado, S. Reversible Thermochromic Polymeric Thin Films Made of Ultrathin 2D Crystals of Coordination Polymers Based on Copper(I)-Thiophenolates. Adv. Funct. Mater. 2018, 28, 1704040. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, Z.-X.; Zhang, W.-W.; Zuo, J.-L.; Ren, X.-M. Dual-emission and thermochromic luminescence alkaline earth metal coordination polymers and their blend films with polyvinylidene fluoride for detecting nitrobenzene vapor. J. Mater. Chem. C 2018, 6, 7030–7041. [Google Scholar] [CrossRef]
- Bruker Axs. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker Axs Ltd: Karlsruhe, Germany, 2008. [Google Scholar]
- Spek, A. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Klafter, J.; Shlesinger, M.F. On the relationship among three theories of relaxation in disordered systems. Proc. Natl. Acad. Sci. USA 1986, 83, 848–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihalcescu, I. Analyse temporelle des mécanismes de luminescence du silicium poreux. Université Joseph Fourier-Grenoble 1, 1994. [Google Scholar]
- Dam, B.V.; Bruhn, B.; Dohnal, G.; Dohnalová, K. Limits of emission quantum yield determination. Aip Adv. 2018, 8, 085313. [Google Scholar]
- Ahn, T.-S.; Al-Kaysi, R.O.; Müller, A.M.; Wentz, K.M.; Bardeen, C.J. Self-absorption correction for solid-state photoluminescence quantum yields obtained from integrating sphere measurements. Rev. Sci. Instrum. 2007, 78, 086105. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.X.; Limpens, R.; Gregorkiewicz, T. Investigating Photoluminescence Quantum Yield of Silicon Nanocrystals Formed in SiOx with Different Initial Si Excess; SPIE: Bellingham, WA, USA, 2015; Volume 9562. [Google Scholar]
- Lim, J.S.; Choi, H.; Lim, I.S.; Park, S.B.; Lee, Y.S.; Kim, S.K. Photodissociation Dynamics of Thiophenol-d1: The Nature of Excited Electronic States along the S−D Bond Dissociation Coordinate. J. Phys. Chem. A 2009, 113, 10410–10416. [Google Scholar] [CrossRef]
- Li, C.-H.; Kui, S.C.F.; Sham, I.H.T.; Chui, S.S.-Y.; Che, C.-M. Luminescent Gold(I) and Copper(I) Phosphane Complexes Containing the 4-Nitrophenylthiolate Ligand: Observation of π→π* Charge-Transfer Emission. Eur. J. Inorg. Chem. 2008, 2008, 2421–2428. [Google Scholar] [CrossRef]
- Monzittu, F.M.; Fernández-Moreira, V.; Lippolis, V.; Arca, M.; Laguna, A.; Gimeno, M.C. Different emissive properties in dithiolate gold(i) complexes as a function of the presence of phenylene spacers. Dalton Trans. 2014, 43, 6212–6220. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Seo, H.J. The Irradiation Induced Valence Changes and the Luminescence Properties of Samarium Ions in Ba2SiO4. J. Electrochem. Soc. 2010, 157, J429–J434. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Zhou, X.; Brites, C.D.S.; Ananias, D.; Lin, Z.; Paz, F.A.A.; Rocha, J.; Huang, W.; Carlos, L.D. Visible-Light Excited Luminescent Thermometer Based on Single Lanthanide Organic Frameworks. Adv. Funct. Mater. 2016, 26, 8677–8684. [Google Scholar] [CrossRef]
- Forward, J.M.; Bohmann, D.; Fackler, J.P.; Staples, R.J. Luminescence Studies of Gold(I) Thiolate Complexes. Inorg. Chem. 1995, 34, 6330–6336. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Absorption and emission spectra of tetrameric gold(I) complexes. Chem. Phys. Lett. 1988, 150, 135–137. [Google Scholar] [CrossRef] [Green Version]
- Kathewad, N.; Kumar, N.; Dasgupta, R.; Ghosh, M.; Pal, S.; Khan, S. The syntheses and photophysical properties of PNP-based Au(i) complexes with strong intramolecular Au⋯Au interactions. Dalton Trans. 2019, 48, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Sakurada, K.; Ito, H. Controlling Mechano- and Seeding-Triggered Single-Crystal-to-Single-Crystal Phase Transition: Molecular Domino with a Disconnection of Aurophilic Bonds. Angew. Chem. Int. Ed. 2013, 52, 12828–12832. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, G.L.; Wrighton, M.S. Organometallic Photochemistry; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Wang, Z.; Xiong, Y.; Kershaw, S.V.; Chen, B.; Yang, X.; Goswami, N.; Lai, W.-F.; Xie, J.; Rogach, A.L. In Situ Fabrication of Flexible, Thermally Stable, Large-Area, Strongly Luminescent Copper Nanocluster/Polymer Composite Films. Chem. Mater. 2017, 29, 10206–10211. [Google Scholar] [CrossRef]
- Wang, M.-S.; Guo, G.-C. Inorganic–organic hybrid white light phosphors. Chem. Commun. 2016, 52, 13194–13204. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselska, O.; Guillou, N.; Ledoux, G.; Huang, C.-C.; Dohnalova Newell, K.; Elkaïm, E.; Fateeva, A.; Demessence, A. A New Lamellar Gold Thiolate Coordination Polymer, [Au(m-SPhCO2H)]n, for the Formation of Luminescent Polymer Composites. Nanomaterials 2019, 9, 1408. https://doi.org/10.3390/nano9101408
Veselska O, Guillou N, Ledoux G, Huang C-C, Dohnalova Newell K, Elkaïm E, Fateeva A, Demessence A. A New Lamellar Gold Thiolate Coordination Polymer, [Au(m-SPhCO2H)]n, for the Formation of Luminescent Polymer Composites. Nanomaterials. 2019; 9(10):1408. https://doi.org/10.3390/nano9101408
Chicago/Turabian StyleVeselska, Oleksandra, Nathalie Guillou, Gilles Ledoux, Chia-Ching Huang, Katerina Dohnalova Newell, Erik Elkaïm, Alexandra Fateeva, and Aude Demessence. 2019. "A New Lamellar Gold Thiolate Coordination Polymer, [Au(m-SPhCO2H)]n, for the Formation of Luminescent Polymer Composites" Nanomaterials 9, no. 10: 1408. https://doi.org/10.3390/nano9101408




